Answer: The percent yield of silver is 86.96 %
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 .....(1)
.....(1)
Given mass of copper = 40.0 g
Molar mass of copper = 63.55 g/mol
Putting values in equation 1, we get:
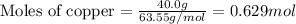
The given chemical equation follows:
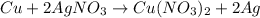
By Stoichiometry of the reaction:
1 mole of copper produces 2 moles of silver
So, 0.629 moles of copper will produce =
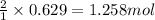 of silver
of silver
Now, calculating the mass of silver from equation 1, we get:
Molar mass of silver = 107.9 g/mol
Moles of silver = 1.258 moles
Putting values in equation 1, we get:
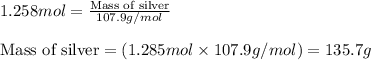
To calculate the percentage yield of silver, we use the equation:
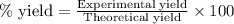
Experimental yield of silver = 118 g
Theoretical yield of silver = 135.7 g
Putting values in above equation, we get:
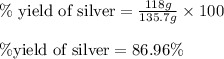
Hence, the percent yield of silver is 86.96 %