Answer:
20 Liters
Step-by-step explanation:
If the pressure is supposed to be constant, one of Charles - Gay Lussac's laws can be used to solve the exercise. His statement says that "the volume of the gas is directly proportional to its temperature at constant pressure", mathematically it would be:
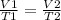
Considering T₁ = 50 ° C; V₁ = 10.0 L; and T₂ = 100 ° C, we can calculate the volume V₂ Clearing it from the equation and replacing the values to perform the calculation:
V2= (V1 x T2) / T1 = (10.0 L x 100°C) / 50 °C = 20 L
Therefore, the gas at 100 ° C will occupy a volume of 20.0 L.