Answer:
116.1 a.m.u.
It is not likely that RCOOH is the pentanoic acid
Step-by-step explanation:
Let's consider the generic neutralization between NaOH and a monoprotic carboxylic acid.
RCOOH(aq) + NaOH(aq) ⇒ RCOONa(aq) + H₂O(l)
The molar ratio of RCOOH to NaOH is 1:1. The moles of RCOOH are:
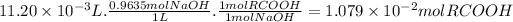
The molar mass of RCOOH is:
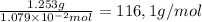
Thus, the molecular weight is 116.1 a.m.u.
Pentanoic acid has the formula C₅H₁₀O₂ with a molecular weight of 102.1 a.m.u. So, it is not likely that RCOOH is the pentanoic acid.