Answer:0.0909 kJ/K
Step-by-step explanation:
Given
Temperature of hot Reservoir
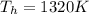
Temperature of cold Reservoir
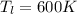
Heat of 100 kJ is transferred form hot reservoir to cold reservoir
Hot Reservoir is Rejecting heat therefore

Heat is added to Reservoir therefore
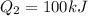
Entropy change for system
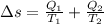
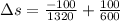
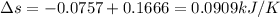
As entropy change is Positive therefore entropy Principle is satisfied