Answer:
11.2 g
Step-by-step explanation:
Given,
Mass of P = 4.89 g
Molar mass of
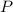 = 30.9738 g/mol
= 30.9738 g/mol
The formula for the calculation of moles is shown below:
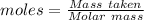
Thus,
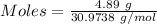
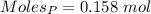
According to the reaction shown below as:-
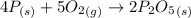
4 moles of P on reaction forms 2 moles of diphosphorus pentoxide
Also,
1 mole of P on reaction forms 2/4 mole of diphosphorus pentoxide
0.158 moles of P on reaction forms
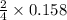 moles of diphosphorus pentoxide
moles of diphosphorus pentoxide
Moles of diphosphorus pentoxide = 0.079 moles
Molar mass of diphosphorus pentoxide = 141.9445 g/mol
Mass = Moles * Molar mass = 0.079 moles * 141.9445 g/mol = 11.2 g