“Aluminum” and “Nitrogen” form an ionic bond.
Option: D
Step-by-step explanation:
Aluminum and nitrogen are most likely to form an “ionic bond” among given pairs of elements in form of aluminum nitride. AlN is partially covalent and also show ionic characteristics as N needs 3 electrons to fill outer shell and Al has 3 electrons in valence shell. Hence
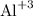 is cation and
is cation and
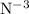 is anion and have “electrostatic force” of attraction between these oppositely charged ions. Here ionic behavior is seen as nitrogen is more “electronegative” hence attract the shared pair of electrons which is earlier responsible for partial covalent bond formation.
is anion and have “electrostatic force” of attraction between these oppositely charged ions. Here ionic behavior is seen as nitrogen is more “electronegative” hence attract the shared pair of electrons which is earlier responsible for partial covalent bond formation.