Answer:
The vapor pressure of water at 26 °C = 26.25 atm
Step-by-step explanation:
Using Boyle's law
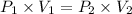
Given ,
V₁ = 2.00 L
V₂ = 1.93 L
P₁ = ?
P₂ = 750 torr
Using above equation as:
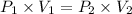

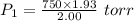
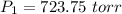
Vapor pressure = Total pressure - Partial pressure of oxygen gas = 750 - 723.75 atm = 26.25 atm
The vapor pressure of water at 26 °C = 26.25 atm