Answer: The expression of
 for calcium fluoride is
for calcium fluoride is
![K_(sp)=[Ca^(2+)][2F^-]^2](https://img.qammunity.org/2020/formulas/chemistry/high-school/86jp8fpt7udlc5zdlu66w7lg02loifpzaz.png)
Step-by-step explanation:
Solubility product is defined as the product of concentration of ions present in a solution each raised to the power its stoichiometric ratio.
The given chemical equation follows:
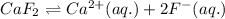
1 mole of calcium fluoride produces 1 mole of calcium ions and 2 moles of fluorine ions.
The expression of
 for above equation follows:
for above equation follows:
![K_(sp)=[Ca^(2+)][2F^-]^2](https://img.qammunity.org/2020/formulas/chemistry/high-school/86jp8fpt7udlc5zdlu66w7lg02loifpzaz.png)
Hence, the expression of
 for calcium fluoride is
for calcium fluoride is
![K_(sp)=[Ca^(2+)][2F^-]^2](https://img.qammunity.org/2020/formulas/chemistry/high-school/86jp8fpt7udlc5zdlu66w7lg02loifpzaz.png)