Answer:
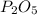 has molar mass of 142 g.
has molar mass of 142 g.
Explanation:
Firstly we will find out the atomic weight of the elements of the compound.
Mg = 24 P = 31 Al = 27 Cl = 35 Ba = 137 O = 16
Now 1st compound is
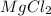 , in this compound there is 1 atom of Magnesium and 2 atom of Chlorine.
, in this compound there is 1 atom of Magnesium and 2 atom of Chlorine.
So atomic weight of
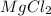 =
=

2nd compound is
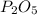 , in this compound there is 2 atom of Phosphorus and 5 atom of Oxygen.
, in this compound there is 2 atom of Phosphorus and 5 atom of Oxygen.
So atomic weight of
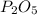 =
=
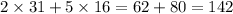 .
.
3rd compound is
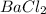 , in this compound there is 1 atom of Barium and 2 atom of Chlorine.
, in this compound there is 1 atom of Barium and 2 atom of Chlorine.
So atomic weight of
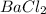 =
=
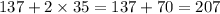
4th compound is
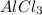 , in this compound there is 1 atom of Aluminium and 3 atom of Chlorine.
, in this compound there is 1 atom of Aluminium and 3 atom of Chlorine.
So atomic weight of
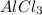 =
=
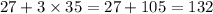
Hence the substance having molar mass of 142 g is
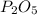 .
.