To solve this problem it is necessary to apply an energy balance equation in each of the states to assess what their respective relationship is.
By definition the energy balance is simply given by the change between the two states:
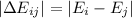
Our states are given by
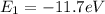

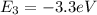
In this way the energy balance for the states would be given by,
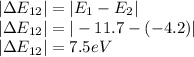
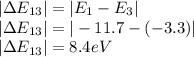
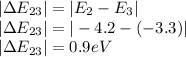
Therefore the states of energy would be
Lowest : 0.9eV
Middle :7.5eV
Highest: 8.4eV