Answer:
The water must be added to 1 liter of a 5% saline solution to get a 2% saline solution is 1.5 litres
Step-by-step explanation:
We assume that the quantity of water added to be x litres. The quantity of saline in the existing solution is 5% of 1litre = 0.05 litres, with the addition of water, the quantity of new solution becomes (1 + x) litres. As per the problem, the percentage of saline in new solution should be equal to 2%. Therefore,
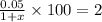
So, 5 = 2(1+x)
2x = 5 - 2
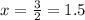
So, 1.5 litres water should be added to make the 1litre 5% saline solution a 2% saline solution.