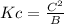Answer:
Part A:
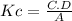
Part B:
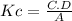
Part C:
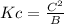
Part D:
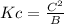
Step-by-step explanation:
Write an equilibrium expression for each chemical equation involving one or more solid or liquid reactants or products.
The equilibrium constant (Kc) is equal to the product of the concentration of the products raised to their stoichiometric coefficients divided by the product of the concentration of the reactants raised to their stoichiometric coefficients. It only includes gases and aqueous species (not liquids or solids).
Part A: HCHO₂(aq) + H₂O(l) ⇌ H₃O⁺(aq) + CHO₂⁻(aq)
Use A for [HCHO₂], B for [H₂O], C for [H₃O⁺], D for [CHO₂⁻].
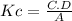
PART B: CO₃²⁻(aq) + H₂O(l) ⇌ HCO₃⁻(aq) + OH⁻(aq)
Use A for [CO₃²⁻], B for [H₂O], C for [HCO₃⁻], D for [OH⁻].
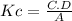
PART C: 2 C(s) + O₂(g) ⇌ 2 CO(g)
Use A for [C], B for [O₂], C for [CO].
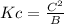
PART D: C(s) + CO₂(g) ⇌ 2 CO(g)
Use A for [C], B for [CO₂], C for [CO].