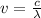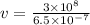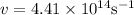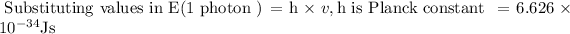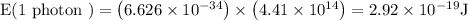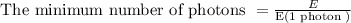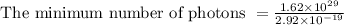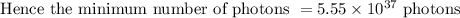Answer:
(a)The balanced thermochemical equation for formation of 1.00 mol of glucose is:
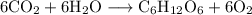
(b) The minimum number of photons with λ = 680 nm needed to prepare 1.00 mol of glucose is
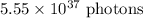
Step-by-step explanation:
(a) Both side of reaction have equal number of elements therefore number of reactant is equal to number of product hence following balanced equation is achieved :
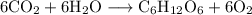
(b) According to theory of special relativity which expresses fact in equation about mass and energy is:
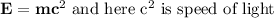
Here λ = 680 nm,
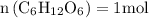
Molar mass of glucose = 180.156 g/mol
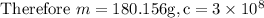
Substituting values in above equation
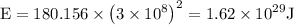
Hence it is known that
E (1 photon) = h × v