Answer:
1. C₄H₁₀ + ¹³/₂O₂ → 4CO₂ + 5H₂O
2. V = 596L
Step-by-step explanation:
Butane (C₄H₁₀) reacts with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O) thus:
C₄H₁₀ + O₂ → CO₂ + H₂O
1. The balanced chemical equation is:
C₄H₁₀ + ¹³/₂O₂ → 4CO₂ + 5H₂O
2. 0,360kg of butane are:
360g×
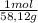 =6,19moles of butane
=6,19moles of butane
These moles of butane are:
6,19moles of butane×
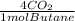 = 24,8 moles CO₂
= 24,8 moles CO₂
Using V=nRT/P
Where:
n are moles (24,8 moles CO₂); R is gas constant (0,082atmL/molK); T is temperature, 20°C (293,15K); and P is pressure (1atm).
Volume (V) is:
V = 596L
I hope it helps!