Answer:
4-
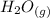
Step-by-step explanation:
Initially, the beaker contains distilled water which is liquid water at room temperature. When the beaker was placed on a hot plate and heat energy was applied the temperature of the system increases gradually until the boiling point was reached. Water generally boils at 100°C and at this temperature, liquid water changes to vapor or gas. In addition, liquid water and steam or water vapor are in equilibrium. Therefore, the presence of water vapor (
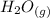 ) indicates that the process is a physical change.
) indicates that the process is a physical change.