Answer: The ionic compound formed is
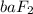 (barium fluoride)
(barium fluoride)
Step-by-step explanation:
Ionic compound is formed by the complete transfer of electrons from 1 atom to another atom. The cation is formed by the loss of electrons by metals and anions are formed by gain of electrons by non metals.
Taking the metal as barium and non-metal as fluorine.
Barium is the 56th element of the periodic table having electronic configuration of
![[Xe]6s^2](https://img.qammunity.org/2020/formulas/chemistry/middle-school/14psnspetimewzf4si7rpt06do00n9r2em.png)
This element will loose 2 electrons and will form
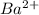 ion
ion
Fluorine is the 9th element of the periodic table having electronic configuration of
![[He]2s^22p^5](https://img.qammunity.org/2020/formulas/chemistry/high-school/rghsvaw5itj4y30un8gnfls77bqlyjxe4p.png)
This element will gain 1 electron and will form
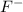 ion
ion
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
Hence, the ionic compound formed is
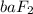 (barium fluoride)
(barium fluoride)