To solve this problem it is necessary to apply the concepts related to heat exchange in the vegetable and water.
By definition the exchange of heat is given by
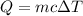
where,
m = mass
c = specific heat
 = Change in temperature
= Change in temperature
Therefore the total heat exchange is given as
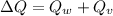
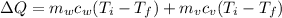
Our values are given as,
Total mass is
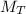 = 200lb ,however the mass of solid vegetable and water is given as,
= 200lb ,however the mass of solid vegetable and water is given as,
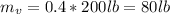
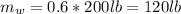
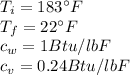
Replacing at our equation we have,
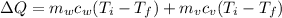
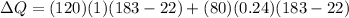
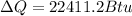
Therefore the heat removed is 22411.2 Btu