To solve the problem it is necessary to apply the concepts related to Byle's Law and Avogadro's Law.
The ideal gas equation would help us find the final solution to the problem, defined by
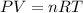
Where,
T= Temperature of the gas
R = Universal as constant
n = number of moles
V = Volume
P = Pressure
For our case we have that the mass of Zn is 2.2g in moles would be
![[tex]Zn = (2.2)/(65)](https://img.qammunity.org/2020/formulas/physics/college/rjxtyus4s804f649l9fde2975p4b07ht9o.png) [/tex]
[/tex]
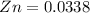
We know that 1 mole of hydrogen gas is proceed by 1 mole of zinc and the result is
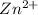 , then Hydrogen can produce the same quantity,
, then Hydrogen can produce the same quantity,
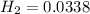
Applying the previous equation we have that
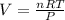
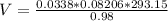
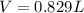
Therefore the volume of hydrogen gas is collected is 0.829L