Answer: The mass of magnesium in given amount of alloy is 0.396 g
Step-by-step explanation:
We are given:
Percent mass of manganese = 4.5 %
Percent mass of copper = 3.5 %
Percent mass of magnesium = 0.45 %
Percent mass of aluminium = (100 - 4.5 - 3.5 - 0.45) = 91.55 %
Mass of alloy = 88 g
We need to calculate the mass of magnesium in 88 g of alloy, we get:
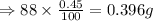
Hence, the mass of magnesium in given amount of alloy is 0.396 g