Answer:
The amount of energy required is 31.1692 kJ .
Step-by-step explanation:
The transitions of water are as follows,
(243K ice to 273K ice to 273K water to 373k water to 373K steam to 393K steam)
The following required data is,
specific heat capacity of ice= 2.1 kJ/kg
specific heat capacity of water= 4.2 kJ/kg
specific heat capacity of ice= 1.996 kJ/kg
specific latent heat of fusion=334 kJ/kg
specific latent heat of vapourisation = 2260 kJ/kg
FORMULAS:-
temperature change=mc(T2-T1)
phase transistion = mL,
where, m=mass , c=specific heat capacity ,L = latent heat of fusion or vapourisation ,(T2-T1)= temparature change
Thus the total amount of heat is,
=mc(T4-T3) + mL + mc(T3-T2) + mL + mc(T2-T1)
=
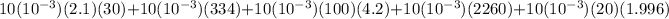
=(0.3)(2.1) + (3.34) + (4.2) + (22.6) + (0.2)(1.996)
=31.1692 kJ.