Answer:
The pressure decreases to 0.4 kilopascals.
Step-by-step explanation:
Given:
Initial volume of the gas is,
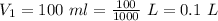
Final volume of the gas is,
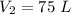
Initial pressure of the gas is,
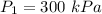
Final pressure of the gas is,
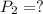
The temperature is constant (isothermal) for the given process.
We know that for a process of constant temperature, pressure of a gas varies inversely with volume. This fact is known as Boyle's Law. So, as per Boyle's Law, the product of pressure and volume of a gas undergoing isothermal process is a constant. Therefore,
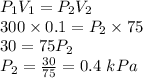
Therefore, the pressure of the gas gets reduced to a value of 0.4 kPa.