Answer:
The temperature of the water stayed at
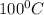 because the temperature of a pure water does not increase at its boiling point,
because the temperature of a pure water does not increase at its boiling point,
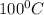 . This is due to latent heat of vaporisation.
. This is due to latent heat of vaporisation.
Step-by-step explanation:
During a change of state of a substance, the temperature does not change due to latent heat of the substance. Latent heat is the amount of heat required to convert a unit mass of a substance from one state to another without a change in its temperature. There are two types of latent heat, latent heat of fusion anf latent heat of vaporisation.
So during the experiment, the water got to its boiling point and continues to boil at this constant temperature until it would change completely to steam at that temperature. After the conversion process, the temperature begins to increase.
The latent heat of vaporisation came into action during the process of boiling the water at constant temperature. That's why the temperature of the water did not increase even after reaching its boiling point (
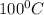 ).
).