Answer:
A. Water would be a gas at room temperature, and
D. Ice would sink in water.
Step-by-step explanation:
There are three types of intermolecular forces: London dispersion forces, dipole-dipole interactions, and hydrogen bonds. The relative strength of these forces depend on the size of the molecule. However, for small molecules like water (three atoms per molecule,) hydrogen bonds would be much stronger than the other two types of forces.
Without hydrogen bonds, water molecules would be held together only with dipole-dipole interactions and London dispersion forces. To get an idea of what that would be like, consider hydrochloric acid
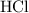 .
.
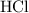 and water
and water
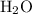 contain about the same number of electrons. The H-Cl bond in
contain about the same number of electrons. The H-Cl bond in
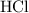 is polar, which allows for dipole-dipole interactions. However, only H-O, H-F, and H-N bonds allow for hydrogen bonding. As a result, there won't be any hydrogen bonding between
is polar, which allows for dipole-dipole interactions. However, only H-O, H-F, and H-N bonds allow for hydrogen bonding. As a result, there won't be any hydrogen bonding between
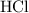 molecules. Without hydrogen bonding,
molecules. Without hydrogen bonding,
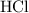 boils at well below
boils at well below
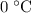 under standard pressure. It is a gas at room temperature under standard pressure. That's about the same as what water molecules would behave (physically) without any hydrogen bonds between them.
under standard pressure. It is a gas at room temperature under standard pressure. That's about the same as what water molecules would behave (physically) without any hydrogen bonds between them.
Also because of hydrogen bonding, the density of ice (solid
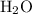 ) is typically greater than that of water (liquid
) is typically greater than that of water (liquid
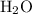 .) When compared to water in its liquid state, there are more hydrogen bondings between molecules of water in its solid state. The hydrogen bonds hold the molecules together to form a lattice. Because of this structure due to hydrogen bondings, the molecules are farther apart than they are in the liquid states. As a result, the density of ice is typically smaller than that of water. That would likely not be the case if there was no hydrogen bondings between water molecules.
.) When compared to water in its liquid state, there are more hydrogen bondings between molecules of water in its solid state. The hydrogen bonds hold the molecules together to form a lattice. Because of this structure due to hydrogen bondings, the molecules are farther apart than they are in the liquid states. As a result, the density of ice is typically smaller than that of water. That would likely not be the case if there was no hydrogen bondings between water molecules.