Answer:
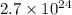 atoms
atoms
Step-by-step explanation:
The given formula of the compound is
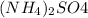
The formula says
Every mole of
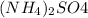 contains
contains
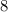 moles of atoms of hydrogen.
moles of atoms of hydrogen.
Given that number of moles of compound is
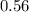
So,the number of moles of hydrogen atoms present is
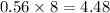
Since each mole has
 atoms,
atoms,
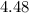 moles has
moles has
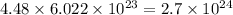 atoms.
atoms.