The complete question is as follows:
Calculate the volume in liters of a
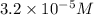 silver(II) oxide solution that contains 200 g of silver(II) oxide . Round your answer to significant digits.
silver(II) oxide solution that contains 200 g of silver(II) oxide . Round your answer to significant digits.
Step-by-step explanation:
Given: Molarity =
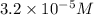 , mass = 200 g
, mass = 200 g
The number of moles is given mass divided by molar mass of substance.
Molar mass of silver(II) oxide
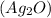 is 231.735 g/mol. Hence,
is 231.735 g/mol. Hence,
No. of moles =
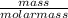
=
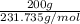
= 0.863 moles
Molarity is the number of moles present in liter of solution. Therefore,
Molarity =
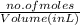
So, volume (in L) =
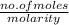
=
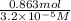
=
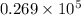 L
L
Thus, volume of given solution is
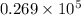 L.
L.