Answer:
999 kJ
Step-by-step explanation:
Given are two reactions with enthalpies Δ
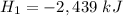 and Δ
and Δ
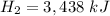
We know enthalpy is an additive property.
Thus the overall chemical equation can be obtained from adding both the two elementary equations.
Therefore the overall enthalpy is the summation of enthalphy change in each equation.
Δ
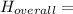 Δ
Δ
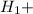 Δ
Δ

Δ
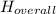
Therefore the overall enthalpy is 999 kJ.