Answer:
81.732KJ
Step-by-step explanation:
Reaction : CaCO₃(s) → CaO(s) + CO₂(g), +556KJ
⇒To decompose 1 mole of CaCO₃, 556KJ of energy is required
The molecular weight of CaCO₃ is 40 + 12 + 3×16
⇒ molecular weight of CaCO₃ = 100 g
∴ 100 g of CaCO₃ requires 556KJ of energy
Need to find out how much energy is required by 14.7 g of CaCO₃
⇒
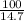 =
=
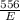
⇒ E =
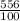 ×14.7 KJ = 81.732 KJ
×14.7 KJ = 81.732 KJ
∴ Energy required to decompose 14.7 g of CaCO₃ is 81.732 KJ