Answer:
E) rate of appearance of C = 0.45 M/s
rate of the reaction = 0.15 M/s
Step-by-step explanation:
2A + B → 3C
Writing rate law for the reaction:
Rate of reaction = -
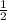
![(d[A])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/biz8d5qf18hrl8osnf4bpjld77ttzt7v96.png) = -
= -
![(d[B])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/m4ouwmor13gqezjctx6qzr52mattgplayf.png) =
=
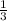
![(d[C])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/b7q0rybhkbrmfrd29bgnzcr1awiqj07kr5.png) → equation 1
→ equation 1
Given that the rate of disappearance of A is 0.3 M/s
⇒ -
![(d[A])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/biz8d5qf18hrl8osnf4bpjld77ttzt7v96.png) = 0.3 M/s
= 0.3 M/s
⇒Rate of reaction = -
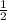
![(d[A])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/biz8d5qf18hrl8osnf4bpjld77ttzt7v96.png) =
=
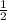 ×0.3 M/s
×0.3 M/s
⇒Rate of reaction = 0.15 M/s
From equation 1,
![(d[C])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/b7q0rybhkbrmfrd29bgnzcr1awiqj07kr5.png) = -
= -
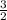
![(d[A])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/biz8d5qf18hrl8osnf4bpjld77ttzt7v96.png) =
=
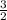 ×0.3 M/s
×0.3 M/s
⇒
![(d[C])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/b7q0rybhkbrmfrd29bgnzcr1awiqj07kr5.png) = 0.45 M/s
= 0.45 M/s
or the rate of appearance of C = 0.45 M/s