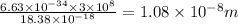Answer:
Step-by-step explanation:
Let
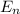 be the energy of the electron in
be the energy of the electron in
 th orbit.
th orbit.
According to Bohr's model,
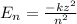
where
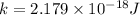
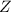 is the atomic number
is the atomic number
 is the orbit number.
is the orbit number.
Given,
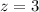
Energy required for transition from
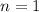 to
to
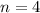 is
is
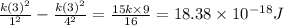
Since,wave length is
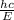
where
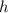 is the plancks constant.
is the plancks constant.
 is the speed of light.
is the speed of light.
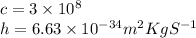
So,wave length is