Step-by-step explanation:
The given data is as follows.
Molecular weight of azulene = 128 g/mol
Hence, calculate the number of moles as follows.
No. of moles =
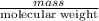
=
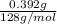
= 0.0030625 mol of azulene
Also,
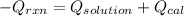
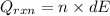
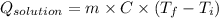
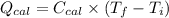
Now, putting the given values as follows.
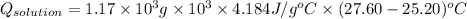
= 11748.67 J
So,
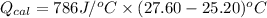
= 1886.4 J
Therefore, heat of reaction will be calculated as follows.
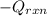 = (11748.67 + 1886.4) J
= (11748.67 + 1886.4) J
= 13635.07 J
As,
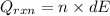
13635.07 J =

dE =
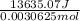
= 4452267.75 J/mol
or, = 4452.26 kJ/mol (as 1 kJ = 1000 J)
Thus, we can conclude that
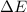 for the given combustion reaction per mole of azulene burned is 4452.26 kJ/mol.
for the given combustion reaction per mole of azulene burned is 4452.26 kJ/mol.