Answer: The student should pour out 92.068 cm³ of diethylamine.
Step-by-step explanation:
The equation for density is:
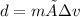
We are given the density and mass of a liquid and asked to calculate the
volume of the liquid. We rearrange the equation to give
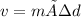
v= 65.0 g÷0.706 g.cm³ = 92.068 cm³
The grams cancel, thus the unit left is cm³.