Answer:
0.41mole
Step-by-step explanation:
Given quantity:
Number of atoms of Na = 4.92 x 10²² atoms
Number of moles in this atom;
6.02 x 10²³ atoms is found in 1 mole of a substance
4.92 x 10²² atoms will have
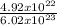 = 0.82 x 10⁻¹ = 0.082mole
= 0.82 x 10⁻¹ = 0.082mole
So;
In Na₂O;
2 mole of Na is found in 1 mole of Na₂O
0.82mole of Na will be found in
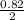 mole of Na₂O = 0.41mole of Na₂O
mole of Na₂O = 0.41mole of Na₂O