Answer:
water is in the vapor state,
Step-by-step explanation:
We must use calorimetry equations to find the final water temperatures. We assume that all energy is transformed into heat
E = Q₁ +
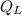
Where Q1 is the heat required to bring water from the current temperature to the boiling point
Q₁ = m
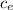 (
(
 -T₀)
-T₀)
Q₁ = 50 4180 (100 - 37)
Q₁ = 1.317 10⁷ J
Let's calculate the energy so that all the water changes state
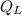 = m L
= m L
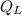 = 50 2,256 106
= 50 2,256 106
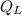 = 1,128 10⁸ J
= 1,128 10⁸ J
Let's look for the energy needed to convert all the water into steam is
Qt = Q₁ +
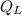
Qt = 1.317 107 + 11.28 107
Qt = 12,597 10⁷ J
Let's calculate how much energy is left to heat the water vapor
ΔE = E - Qt
ΔE = 10¹⁰ - 12,597 10⁷
ΔE = 1000 107 - 12,597 107
ΔE = 987.4 10⁷ J
With this energy we heat the steam, clear the final temperature
Q = ΔE = m
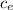 (
(
 -To)
-To)
(
 -T₀) = ΔE / m
-T₀) = ΔE / m
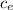
 = T₀ + ΔE / m
= T₀ + ΔE / m
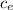
 = 100 + 987.4 10⁷ / (50 1970)
= 100 + 987.4 10⁷ / (50 1970)
 = 100 + 1,002 10⁵
= 100 + 1,002 10⁵
 = 1,003 10⁵ ° C
= 1,003 10⁵ ° C
This result indicates that the water is in the vapor state, in realizing at this temperature the water will be dissociated into its hydrogen and oxygen components