Answer:
3.41 x10⁶ torr
Step-by-step explanation:
To solve this problem we need to remember the equivalency:
1 torr = 133.322 Pa
Then we can proceed to convert 4.55×10⁸ Pa into torr. To do that we just need to multiply that value by a fraction number, putting the unit that we want to convert from in the denominator, and the value we want to convert to in the numerator:
4.55x10⁸ Pa *
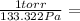 3.41 x10⁶ torr
3.41 x10⁶ torr