Answer:

Step-by-step explanation:
1) Key concepts and notation
For this case we can use the Einstein's formula for the energy of a photon. The original formula from Einstein's is:
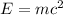
Also Planck's find and equation for the energy of a photon, given by:
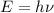
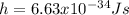 representing the Planck's constant
representing the Planck's constant
f= the frequency
Based on the above formula, the energy of a photon depends only on its wavelength and frequency. The energy is proportional to
![\lambda f\tex].</p><p>[tex]E_A=2.1x10^(-18)J](https://img.qammunity.org/2020/formulas/chemistry/high-school/v40ni5awmxzhu4w4bxjw59ulxh7c74lax1.png) (given from the problem).
(given from the problem).
2) Formulas to apply
The following relationship is important:
 (1)
(1)
Where
 is the speed of the light
is the speed of the light
Solving f from equation (1) we got
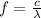 (2)
(2)
3) Apply the formulas
We can find the energy for each source on the following way:

Replacing the value given, we got:
 (3)
(3)
After this solving for
 from equation (3) we got:
from equation (3) we got:
 (4)
(4)
Similarly for the energy source B we have:
 (5)
(5)
For the next step we can apply the condition given, and we got:
 (6)
(6)
Replacing the condition on equation (6) into equation (5):
 (7)
(7)
And now, we can replace equation (4) into equation (7):
 (7)
(7)
So the final answer would be
 .
.