Answer:
0.0826 of the volume in the container is actually occupied by Ar atoms
Step-by-step explanation:
First we find volume occupied by 1 mole the gas using Ideal gas equation
PV = nRT
Given:
P - 230 atm
R - 0.0821 atmL/molK
T - 0 C or 273 K
n - 1
V - Vg (Volume occupied by 1 mole of gas)
Therefore,
Vg = 1 * 0.0821 * 273 / 230 = 0.09745 L
Then we find the volume actually occupied by 1 mole of Ar atoms - Va
b = 4Va
Therefore,
Va = 0.0322 / 4 = 0.00805 L
The fraction of the volume in the container actually occupied by Ar atoms is
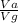
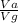 =
=
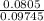 = 0.0826
= 0.0826
Therefore, 0.0826 of the volume in the container is actually occupied by Ar atoms