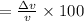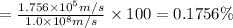Answer:
Percentage of uncertainty in average speed of an electron is 0.1756%.
Step-by-step explanation:
Using Heisenberg uncertainty principle:
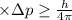
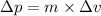
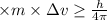
Δx = Uncertainty in position
Δp = Uncertainty in momentum
Δv = Uncertainty in average speed
h = Planck's constant =
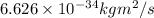
m = mass of electron =
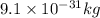
We have
Δx = 2 × 165 pm = 330 pm =
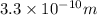
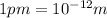
Average orbital speed of electron = v =
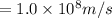
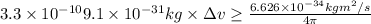
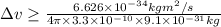
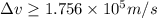
Percentage of uncertainty in average speed: