Answer:
0.094 kg
Step-by-step explanation:
Latent heat of vaporization of
 at 37°C is
at 37°C is
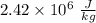 .
.
When the sweat on our body evaporates, it absorbs energy from our body to overcome it's Latent heat of vaporisation. Thus our body cools down when sweat evaporates.
So, Energy absorbed by sweat to evaporate = Energy lost by body
Specific heat capacity of human body =
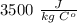 . Jogger weights 60.4 kg. Body temperature decreases by
. Jogger weights 60.4 kg. Body temperature decreases by
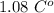
Energy absorbed from body =
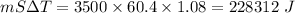
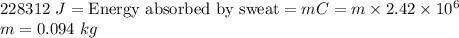
∴ 0.094 kg of sweat has evaporated from the body.