Answer:
1.32 x 10²⁴
Step-by-step explanation:
Given parameters:
Volume of carbon tetrachloride = 49.3L
Unknown:
Number of atoms at STP = ?
Solution:
To solve this problem;
At STP;
1 mole of a substance occupies 22.4L ;
So;
Let us find the number of moles;
22.4L = 1 mole
49.3L will give
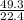 = 2.2mole
= 2.2mole
So;
1 mole of substance contains 6.02 x 10²³ atoms
2.2 mole of Carbon tetrachloride = 2.2 x 6.02 x 10²³ = 1.32 x 10²⁴