If the atoms that share electrons have an unequal attraction for electrons, the bond is called a Polar covalent bond.
Step-by-step explanation:
A covalent chemical bond is formed in case of two different non-metals when one or more electron pairs are shared between bonding atoms. A difference in electronegativity of subsequent atoms of a covalent bond leads to formation of a small net charge around nucleus of each atom, pulling the shared electrons to one side of the bond, to the nucleus which has higher electronegativity.
HCl is an example of polar covalent bond and the HCl bond has Chlorine more electronegative. The bonding electrons are more close to Cl than H and hence Cl is partially negatively charged than H which has partial positive charge (HCl bond :
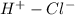 ). When electrons shared in a covalent bond have equal attraction, the bond is a Non-Polar covalent bond.
). When electrons shared in a covalent bond have equal attraction, the bond is a Non-Polar covalent bond.