Answer:
827 mL
Step-by-step explanation:
To answer this question we use the definition of Molarity:
Molarity = mol / L
[Cl⁻] = mol Cl⁻ / L
Now we calculate the moles of Cl⁻ present in 42.0 g of MgCl₂⋅6H₂O:
Molar mass of MgCl₂⋅6H₂O = 24.3 + 2*35.45 + 6*18 = 203.2 g/mol
moles of Cl⁻ = 42.0 g MgCl₂⋅6H₂O ÷ 203.2 g/mol *
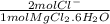 = 0.4134 mol Cl⁻
= 0.4134 mol Cl⁻
Finally we use the definition of molarity to calculate the volume:
0.500 M = 0.4134 mol Cl⁻ / xL
xL = 0.827 L = 827 mL