Step-by-step explanation:
The number of electron density regions that encircle an atom is calculated by the steric number, which also defines the hybridisation of the central atom. In that scenario, there are six hybridised orbitals in the central atom, leading to a
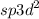 hybridization.
hybridization.
I am joyous to assist you anytime.