Answer:
18.7L
Step-by-step explanation:
To solve this problem, we first find the number of moles the given number of atoms of He represents.
Number of atoms of He = 5.03 x 10²³ atoms
So;
6.02 x 10²³ atoms will contain 1 mole of He
5.03 x 10²³ atoms will have
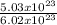 = 0.84mole of He
= 0.84mole of He
Now, the volume at STP;
1 mole of He will occupy 22.4L at STP
0.84 mole of He will occupy 0.84 x 22.4 = 18.7L at STP