Answer:
2
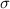 bonds and 2
bonds and 2
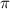 bonds
bonds
Step-by-step explanation:
If we consider the the bonding in the
 molecule:
molecule:
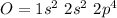
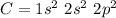
Thus carbon forms double bonds with oxygen:
O = C = O
Now,
We know that double bond comprises of a
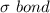 and a
and a
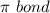
Since, in the
 , there are 2 double bonds thus there are 2
, there are 2 double bonds thus there are 2
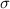 bonds and 2
bonds and 2
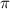 bonds in the molecules.
bonds in the molecules.