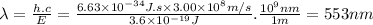Answer:
553 nm
Step-by-step explanation:
When an electron from O absorbs radiation with an energy (E) of 3.6 × 10⁻¹⁹ J, it is excited from orbital 2p to orbital 3s. The wavelength (λ) associated with that radiation can be calculated using the Planck-Einstein equation.
E = h. ν = h . c . λ⁻¹
where,
h is the Planck's constant
c is the speed of light
ν is the frequency