The total pressure that is exerted by the gases : 5.37 atm
Further explanation
Given
Partial pressure of the gas
Required
The total pressure
Solution
Dalton's law of partial pressures stated that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases
Can be formulated:
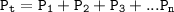
P₁=3 atm
P₂=1.8 atm
P₃=0.29 atm
P₄=0.18 atm
P₅=0.1 atm
P total :
Pt = 3 + 1.8 + 0.29 + 0.18 + 0.1
Pt = 5.37 atm