Answer:
The total cost is 1.25 dollars.
Step-by-step explanation:
The reaction between HCl and CaCO₃ is giving by:
2HCl(aq) + CaCO₃(s) → CaCl₂(aq) + CO₂(g) + H₂O(l) (1)
0.500L M: 100.01g/mol
0.400M
According to equation (1), 2 moles of HCl react with 1 mol of CaCO₃, so to neutralize HCl, we need the next amount of CaCO₃:
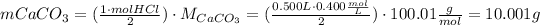
The CaCO₃ mass of each tablet is:
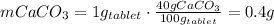
Hence, the number of tablets that we need to neutralize the HCl is:
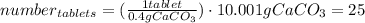
Finally, if every 80 tablets costs 4.00 dollars, 25 tablets will cost:
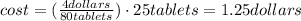
So, the total cost to neutralize the HCl is 1.25 dollars.
I hope it helps you!