Answer:
1.18 × 10⁷ c
Iron is the anode and zinc is the cathode.
Step-by-step explanation:
Let's consider the reduction of Zn²⁺.
Zn²⁺(aq) + 2 e⁻ ⇒ Zn(s)
How many coulombs of charge are needed to produce 61.2 mol of solid zinc?
We can establish the following relations:
- When 2 moles of electrons circulate, 1 mol of Zn is produced.
- 1 mole of electrons have a charge of 96468 c (Faraday's constant).
Then, for 61.2 mol of Zn:
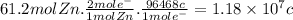
Identify the anode and cathode when plating an iron nail with zinc.
The anode is where the oxidation takes place and the cathode is where the reduction takes place.
Anode (oxidation): Fe(s) ⇒ Fe²⁺(aq) + 2 e⁻
Cathode (reduction): Zn²⁺(aq) + 2 e⁻ ⇒ Zn(s)