Answer:
a) 24.85 grams of sodium sulfate is needed.
b) Mass of 0.00202 moles of chloride ions:
Mass percentage of chloride ion present in the sample is 0.7171%.
Step-by-step explanation:
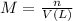
m = Molarity of the solution
n = moles of compound
V = volume of the solution in L.
a) Moles of sodium sulfate = n
Molarity of the solution , M= 0.500 M
Volume of the solution = V = 350.0 mL = 0.3500 L
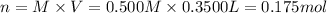
Mass of 0.175 moles of sodium sulfate = 0.175 mol × 142 g/mol = 24.85 g
24.85 grams of sodium sulfate is needed.
b) Moles of silver ion = n
Molarity of the silver ions = M = 0.100 M
Volume of the solution = V = 20.2 mL = 0.0202 L
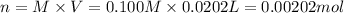
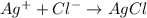
According to reaction , 1 mole of silver ions reacts with 1 mole of chloride ions.
Then 0.00202 moles of silver ions will react with :
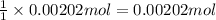 of chloride ions.
of chloride ions.
Mass of 0.00202 moles of chloride ions:
0.00202 mol × 35.5 g/mol = 0.07171 g
Mass percentage of chloride ions in 10.0 grams of water:
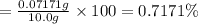
Mass percentage of chloride ion present in the sample is 0.7171%.