Answer:
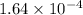 moles of O₂ would be consumed in 1 hr by a 5.4 g cockroach moving at this speed.
moles of O₂ would be consumed in 1 hr by a 5.4 g cockroach moving at this speed.
Step-by-step explanation:
Volume of oxygen gas consumed by the cockroach in an hour = V
V = 0.76 mL = 0.00076 L
Pressure of the oxygen gas = P = 1 atm
Temperature of the gas = T = 32°C = 305.15 K
Moles of oxygen gas consumed by cockroach in an hour = n
 (Using ideal gas law)
(Using ideal gas law)
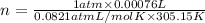
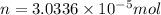
Moles of oxygen gas consumed by 1 gram of mass of an insect = n
Total moles of oxygen gas consumed by 5.4 grams of an insect is:
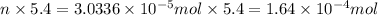
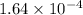 moles of O₂ would be consumed in 1 hr by a 5.4 g cockroach moving at this speed.
moles of O₂ would be consumed in 1 hr by a 5.4 g cockroach moving at this speed.